Click here to see all images
July, 2017
Case of the Month
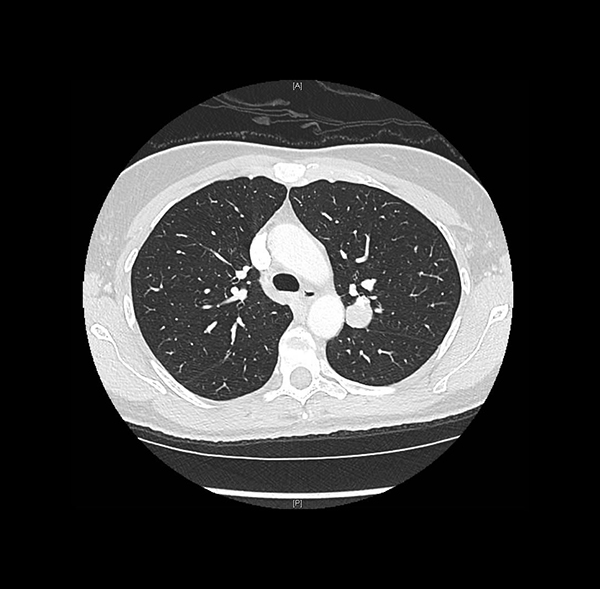
Clinical History: A 47-year-old woman, former smoker, was found to have an incidental lung nodule during the workup of an appendiceal adenocarcinoma ex-goblet cell carcinoid. The chest CT showed a round, well-circumscribed solid nodule in the posterior left upper lobe abutting the major fissure, measuring 2.0 cm (Figure 1). A trans-thoracic core needle biopsy was performed (Figures 2-6; Fig 3: pancytokeratin, Fig 4: epithelial membrane antigen, Fig 5: TTF-1, Fig 6: napsin A).
Quiz:
This tumor is currently thought to be derived from what type of primitive cell?
- Histiocytes
- Blood vessels
- Neuroendocrine cells
- Primitive pneumocytes
- Mesothelial cells
Q2. What pattern is not typically associated with this tumor?
- Sclerotic
- Papillary
- Rosettes
- Solid
- Hemorrhagic
Q3. The two cell types that comprise this entity show overlapping but distinct immunohistochemical profiles. Which immunostains tend to be diffusely positive in both cell types?
- CAM5.2
- EMA and TTF-1
- Pan-cytokeratin
- Napsin A and CK7
- SMA and Chromogranin
Answers to Quiz
Q2. C
Q3. B
Diagnosis
Discussion
This case illustrates the difficulty of diagnosis with limited material resulting in a broad differential. The typical differential for sclerosing pneumocytoma with solid pattern includes carcinoid tumors. For those with papillary pattern the primary differential is adenocarcinoma with papillary growth pattern. The differential for lesions with prominent sclerosing pattern and abundant stromal hyalinization includes other neoplasms with prominent myxohyaline stroma and bland cells, including epithelioid hemangioendothelioma and salivary-type neoplasms. Other vascular neoplasms may enter the differential diagnosis of lesions with a hemorrhagic pattern. Sclerosing pneumocytomas are consistently negative for synaptophysin, chromogranin, and vascular markers such as ERG. The distinctive immunoprofile of the round cells (TTF+/Napsin-/EMA+/CK-) helps distinguish these lesions from adenocarcinoma.
References
Kim KH, Sul HJ, Kang DY. Sclerosing hemangioma with lymph node metastasis. Yonsei Med J 2003;44:150-4.
Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956;9: 53-75.
Schmidt LA, Myers JL, McHugh JB. Napsin A is differentially expressed in sclerosing hemangiomas of the lung. Arch Pathol Lab Med 2012;136:1580-4.
Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60.
Contributors
Thoracic Pathology research fellow
Department of Pathology
Memorial Sloan Kettering Cancer Center, New York, NY, USA
Natasha Rekhtman, M.D., Ph.D.
Thoracic Pathology Attending
Department of Pathology
Memorial Sloan Kettering Cancer Center, New York, NY, USA