Click here to see all images
August, 2018
Case of the Month
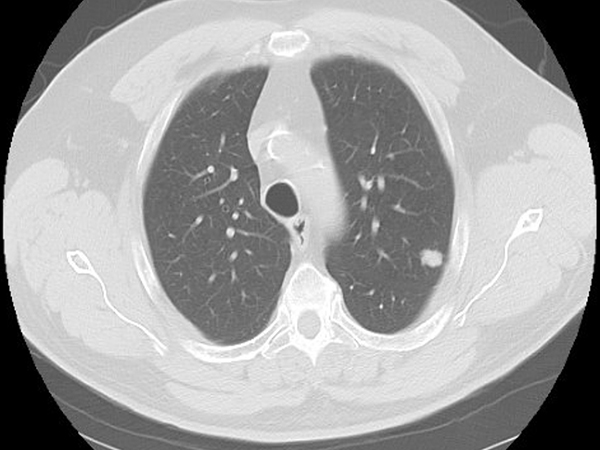
Clinical History: A 68-year-old man was found to have a suspicious left upper lobe nodule on a cancer screening chest CT (Figure 1). He was a former smoker. The patient underwent a VATS left upper lobe segmentectomy. Pathologic review demonstrated two distinct morphologies within the tumor (Figure 2). The periphery of the tumor was an adenocarcinoma with micropapillary and acinar growth patterns (Figure 3) whereas the center demonstrated a solid growth pattern composed of cords and nests of cells with moderate cytoplasm, pleomorphic nuclei with speckled chromatin and prominent nucleoli, areas of necrosis, and mitotic rate of up to 11 mitoses per 10 HPF (Figure 4). Immunohistochemical characterization of the tumor area corresponding to Figure 2 by TTF-1 (Figure 5), synaptophysin (Figure 6), and napsin A (Figure 7) is shown.
Quiz:
Q1. What is the appropriate histologic diagnosis of this tumor (WHO classification)?
- Combined large cell neuroendocrine carcinoma - lung adenocarcinoma
- Adenocarcinoma with concurrent solid basaloid squamous cell carcinoma
- Adenocarcinoma with concurrent atypical carcinoid tumor
- NSCLC with neuroendocrine features
Q2. Which of the following immunohistochemical stains is most often negative in pulmonary neuroendocrine tumors?
- Pan-cytokeratin
- TTF-1
- Napsin A
- CD56/NCAM
Q3. Mutations in which of the following set of genes are frequently seen in small cell carcinoma as well as a subset of large cell neuroendocrine carcinoma?
- ALK , ROS1, EGFR
- RB, TP53
- MET Exon 14, MYC, TET1
- BRAF, KRAS, PDL1
Answers to Quiz
Q2. C
Q3. B
Diagnosis
Discussion
The mixture of LCNEC with any amount of other distinct NSCLC such as adenocarcinoma, giant cell carcinoma, squamous cell carcinoma, or spindle cell carcinoma should be reported as a combined large cell neuroendocrine carcinoma according to the WHO (2015 edition). As seen in Figures 6 and 7, the LCNEC component stains convincingly with a neuroendocrine marker but not napsin A whereas the adenocarcinoma component stains diffusely with napsin A. This is a nice example of a case in which the morphologic and immunophenotypic boundaries between the two components are distinct, with clear-cut morphologic features of neuroendocrine differentiation on H&E in the LCNEC component. This would be in contrast to the occasional findings of pulmonary squamous cell carcinoma or adenocarcinoma which lack neuroendocrine morphology yet show positivity for neuroendocrine markers - these tumors are characterized as NSCLC with neuroendocrine differentiation. Classification of these tumors should be based on the type of NSCLC with a comment on the positive neuroendocrine markers. It is not recommended to routinely perform neuroendocrine markers on tumors in the absence of neuroendocrine morphologic features on H&E.
There is significant clinical and histopathologic overlap between LCNEC and other aggressive pulmonary tumors including small cell carcinoma and lung adenocarcinoma. Recent studies have identified three distinct molecular subtypes of histologically-defined LCNEC by next-generation sequencing: adenocarcinoma-like LCNEC, small cell-like LCNEC, and carcinoid-like LCNEC. The adenocarcinoma-like molecular subtype of LCNECs displays frequent KRAS and STK11 mutations (and napsin A positivity), which are also seen in lung adenocarcinomas. The small cell-like molecular subtype of LCNEC lacks KRAS and STK11 mutations but rather harbors the RB1 and TP53 alterations that characterize a majority of small cell lung cancers. The rare carcinoid-like molecular subtype of LCNEC is characterized by inactivating MEN1 mutations, which can be seen in conventional carcinoids. Because of the limited number of cases in this last group, the authors of these studies stressed the importance of mitotic activity, proliferation index, and the presence of necrosis to distinguish LCNEC from carcinoid tumors. Molecular subtyping of LCNEC may lead to significant changes in future classification of neuroendocrine tumors and their clinical management.
Take home message for trainees: LCNECs may overlap histologically and immunohistochemically with both small cell carcinoma and other types of NSCLC. LCNECs that contain a distinct component of another NSCLC type should be classified as combined large cell neuroendocrine carcinoma.
References
Zhang C, Schmidt LA, Myers JL. Evaluation of napsin A, TTF-1, p63, p40, and CK5/6 immunohistochemical stains in pulmonary neuroendocrine tumors. Am J Clin Pathol. 2014;142:320-4.
Travis WD, Brambilla E, Burke AP, et al, editors. WHO classification of tumours of the lung, pleura, thymus and heart. 4th edition. Lyon (France): World Health Organization; 2015.
Rekhtman N, Pietanza MC, Ladanyi M. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-like and Non-Small Cell Carcinoma-like Subsets. Clin Cancer Res. 2016;22:3618-29.
Rekhtman N, Pietanza CM, Joubert P. Pulmonary large cell neuroendocrine carcinoma with adenocarcinoma-like features: napsin A expression and genomic alterations. Mod Pathol. 2018;31:111-121.
Contributors
Paul VanderLaan MD, PhD Director of Thoracic Pathology, Beth Israel Deaconess Medical Center Assistant Professor of Pathology, Harvard Medical School