Click here to see all images
June, 2018
Case of the Month
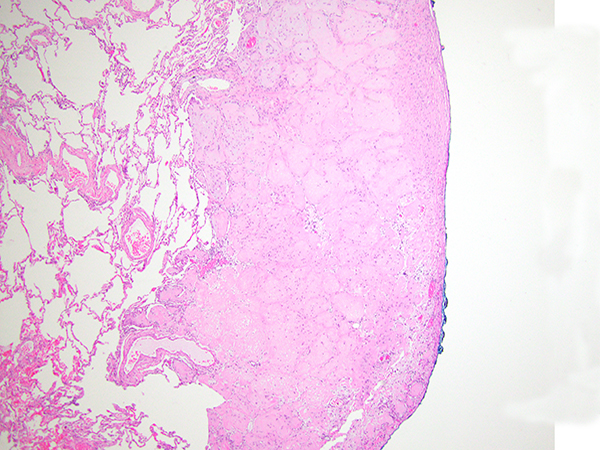
Clinical History: A 36-year-old woman with a history of sarcoidosis diagnosed on skin biopsy presented to the pulmonary clinic. A PET/CT scan performed to evaluate for lung involvement by sarcoidosis showed multiple bilateral well-defined non-PET avid pulmonary nodules lacking lymphangitic spread (Figure 1). No mediastinal lymphadenopathy was noted. Given the unclear nature of the nodules, VATS wedge biopsy was performed. Representative images of the right middle lobe wedge biopsy and nodules are shown in Figures 2 through 5.
Quiz:
Q1. This neoplasm occurs most frequently in:
- Elderly males
- Children and young adults regardless of gender
- Middle aged women
- Middle aged men
- Elderly females
Q2. Typically, by immunohistochemistry, this neoplasm expresses:
- CD31 and ERG
- SMA and desmin
- S100
- TTF-1
- p40
Q3. Which of the following genetic alterations occurs in the majority of examples of this tumor?
- EGFR mutation
- ALK translocation
- BRAF V600E mutation
- DICER mutation
- t(1,3) translocation resulting in the WWTR1-CAMTA1 fusion gene
Answers to Quiz
Q2. A
Q3. E
Diagnosis
Discussion
The wedge biopsy of the right middle lobe showed multiple circumscribed nodules composed of bland epithelioid cells with abundant eosinophilic cytoplasm, protruding into the alveolar spaces with no disruption of the architecture and lack of reactivity of the pneumocytes (Figures 2-5). The nodules appeared hyalinized with cellular accentuation in the periphery and focally myxoid stroma. The neoplastic cells showed intracytoplasmic lumina/vacuoles and occasional nuclear inclusions. By immunohistochemistry, the neoplastic cells expressed multiple vascular markers including CD34, CD31, ERG and Fli1, as well as focal expression of low-weight cytokeratins and EMA. Negative stains included TTF-1, p40, Pax8, and CK7. No amyloid was noted on Congo red stain. There was no evidence of granulomatous inflammation.
The histologic features as mentioned above, together with the clinical history, are very characteristic of epithelioid hemangioendothelioma (EHE). This neoplasm is regarded to as a “borderline” neoplasm, with some authors considering it a low-grade sarcoma. Pulmonary EHE occurs predominantly in young to middle age females and it is most often asymptomatic. As mentioned above, radiologically EHE presents as multiple and bilateral small nodules diffusely affecting the lungs and, therefore, is often confused with lung metastasis, particularly leiomyomas and lymphomas. The histologic features are as mentioned above, with the very characteristic neoplastic epithelioid cells with abundant eosinophilic cytoplasm and the presence of cytoplasmic vacuoles/lumina often containing red blood cells (so-called “blister” cells) and hyalinized to myxoid stroma. EHE can also extensively involve the pleural surface. Pathologists should make an effort to recognize clues to the vascular nature of this neoplasm in order to avoid confusing it with granulomatous processes (particularly sarcoidosis) or carcinomas. Figure 5 exemplifies an area of granulomatous appearance in this case, that could easily be confused with a granuloma. Recently, the majority of EHEs have been shown to harbor the t(1,3) translocation resulting in the WWTR1-CAMTA1 fusion gene, which can be tested by cytogenetic techniques or FISH studies.
EHE can present with multifocal organ involvement and, many times, it is very challenging to determine if an EHE is of lung origin or a metastasis. Likewise, due to the presence of multiple intrapulmonary nodules, surgical resection is often not an effective treatment. Radiation and chemotherapy have also been shown to be of little value. EHE also often recurs locally and has the potential to metastasize. Although EHE is considered a “borderline” neoplasm, it can be fatal due to local and distal progression of the disease.
Take home message for trainees:
Epithelioid hemangioendotheliomas can be confused with sarcoidosis, amyloidosis and even carcinomas, given their occasional cytokeratin expression. Careful microscopic examination to identify the characteristic “blister” cells (morphologic evidence of vascular differentiation), the hyalinized/myxoid-chondroid stroma and the expression of vascular markers by immunohistochemistry help for the proper classification of this neoplasm.
References
Sardaro A, Bardoscia L, Petruzzelli MF, et al. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 2014;13;8:259.
Woo JH, Kim TJ, Lee KS, et al. Epithelioid hemangioendothelioma in the thorax: Clinicopathologic, CT, PET, and prognostic features. Medicine (Baltimore) 2016;95:e4348.
Contributors
Assistant Professor
University of Pittsburgh Medical Center Department of Pathology/Thoracic Pathology