Click here to see all images
October, 2018
Case of the Month
Clinical History: A 52-year-old man, former smoker, presented with difficulty chewing and swallowing, fatigue and shortness of breath after a fall from a skid-loader. Chest CT showed an anterior mediastinal mass (Figure 1). He subsequently underwent thoracotomy with resection of the mass (Figures 2-6).
Quiz:
Q1. What is the most common paraneoplastic syndrome associated with this tumor?
- Myasthenia gravis
- Peripheral neuropathy
- Rheumatoid arthritis
- SIADH
- Systemic Lupus Erythematosus (SLE)
Q2. True or false? These tumors are malignant neoplasms?
- True
- False
Q3. The differential diagnosis of anterior mediastinal masses includes all but which of the following?
- Hodgkin lymphoma
- Neuroblastoma
- Sclerosing mediastinitis
- Thymoma
- T-lymphoblastic lymphoma
Q4. Which of the following is true regarding this entity?
- The neoplastic cells are of lymphoid lineage
- The lymphoid cells are CD20 positivie
- Hassall corpuscles may be present
- The pale cells are epithelioid histiocytes
- TNM staging is based on tumor size
Answers to Quiz
Q1. A
Q2. A
Q3. B
Q4. C
Q2. A
Q3. B
Q4. C
Diagnosis
Thymoma, WHO typeB1, forming a 6.5 X 6.4 X 4.9 cm mass.
Discussion
Thymomas are rare epithelial neoplasms of the anterior mediastinum (Figure 7, arrow). They are the most common mediastinal tumors in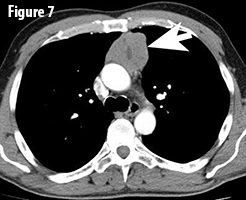 adults with an incidence of 1-5 / million population / year. Thymomas often behave indolently, but are considered to be malignant neoplasms given their potential for aggressive local growth, recurrence, metastasis and potentially even might lead to the death of the patient. It is important to distinguish thymoma from thymic carcinoma, which has an overall worse outcome. Approximately 40% of patients with thymoma have a paraneoplastic or autoimmune syndrome of which myasthenia gravis is the most common. Conversely, 15 to 21% of patients with myasthenia gravis have a thymoma. These syndromes can even be the initial presenting factor as in this case. The prognosis of thymomas is mainly affected by margin status and tumor stage (TNM stage); histologic subtype is also associated with prognosis.
adults with an incidence of 1-5 / million population / year. Thymomas often behave indolently, but are considered to be malignant neoplasms given their potential for aggressive local growth, recurrence, metastasis and potentially even might lead to the death of the patient. It is important to distinguish thymoma from thymic carcinoma, which has an overall worse outcome. Approximately 40% of patients with thymoma have a paraneoplastic or autoimmune syndrome of which myasthenia gravis is the most common. Conversely, 15 to 21% of patients with myasthenia gravis have a thymoma. These syndromes can even be the initial presenting factor as in this case. The prognosis of thymomas is mainly affected by margin status and tumor stage (TNM stage); histologic subtype is also associated with prognosis.
Thymomas are histologically divided according to the WHO classification into five main subtypes (A, AB, B1, B2, B3) based on the cytologic features of the epithelial tumor cells and the relative proportion of tumor cells and immature T-lymphocytes (thymocytes). Type A thymomas are comprised of bland-appearing spindle cells with only very few or no background lymphocytes; type B thymomas are comprised of polygonal epithelial cells. The type B thymomas are further subdivided with decreasing numbers of background lymphocytes from B1 to B3. Type AB is a mixture of type A and type B1 or B2 thymomas.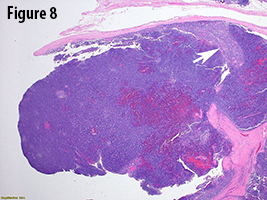
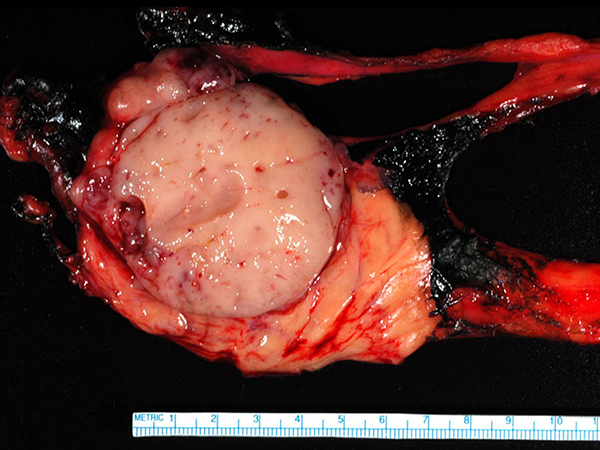
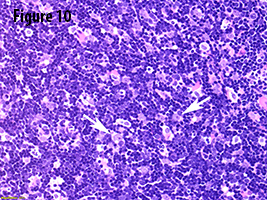
In the current case, gross evaluation (Figure 2) reveals a fleshy, lobulated, tan-pink solid and focally cystic mass with adjacent thymus tissue. Low power microscopic evaluation (Figure 8) shows a dense lymphoid infiltrate with a fibrous capsule and hypocellular fibrous septa. Also in figure 8, an area of paler tissue (arrow), a so-called “medullary island”, is identified along with Hassall corpuscles (Figure 9 arrow). Figure 10 shows scattered epithelioid cells (arrows). These morphologic features are consistent with a WHO type B1 thymoma.
The differential diagnosis of type B1 thymoma includes true thymic hyperplasia, thymic follicular hyperplasia, type B2 thymoma, T-lymphoblastic lymphoma (T-LBL), and thymic carcinoma. While true thymic hyperplasia is characterized by benign thymic parenchyma comprised of medulla and cortex, lacking the fibrous septa and fibrous capsule that are seen in thymomas, the weight of the thymic gland is above the weight that is normal for age. Follicular thymic hyperplasia is characterized by benign thymic gland that is expanded by lymphoid follicles with germinal centers. The differentiation of type B1 from B2 thymoma has historically been somewhat difficult as reflected by the only substantial reproducibility amongst thoracic pathologists. The 2015 WHO classification sought to address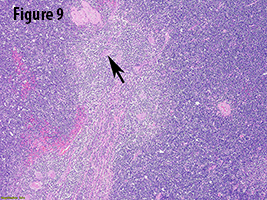 this issue by taking histologic criteria for each thymoma subtype and placing them into categories: “Obligatory/indispensable” or “optional.” The obligatory criteria for type B1 thymomas include abundance of immature T-cells, presence of medullary islands, and absence of epithelial cell clusters (these clusters are present in type B2). Optional criteria include presence of Hassall corpuscles or perivascular spaces. In T-LBL, blasts efface the normal thymic architecture without interspersed neoplastic epithelial cells. A keratin stain will be negative or highlights remaining thymic gland. In contrast to thymomas, thymic carcinomas have a distorted architecture and lack the typical lobulation of thymomas. In addition, they usually show at least focal desmoplasia and often have more pronounced cytologic atypia. While squamous cell carcinomas are the most common subtypes of thymic carcinomas, adenocarcinoma, sarcomatoid carcinomas, mucoepidermoid carcinomas, NUT carcinomas, and lymphoepithelioma-like carcinomas amongst others can also occur.
this issue by taking histologic criteria for each thymoma subtype and placing them into categories: “Obligatory/indispensable” or “optional.” The obligatory criteria for type B1 thymomas include abundance of immature T-cells, presence of medullary islands, and absence of epithelial cell clusters (these clusters are present in type B2). Optional criteria include presence of Hassall corpuscles or perivascular spaces. In T-LBL, blasts efface the normal thymic architecture without interspersed neoplastic epithelial cells. A keratin stain will be negative or highlights remaining thymic gland. In contrast to thymomas, thymic carcinomas have a distorted architecture and lack the typical lobulation of thymomas. In addition, they usually show at least focal desmoplasia and often have more pronounced cytologic atypia. While squamous cell carcinomas are the most common subtypes of thymic carcinomas, adenocarcinoma, sarcomatoid carcinomas, mucoepidermoid carcinomas, NUT carcinomas, and lymphoepithelioma-like carcinomas amongst others can also occur.
Patients with type B1 thymoma have a favorable prognosis with 90% of tumors being completely resectable. Reported 10- and 20-year overall survival rates range from 85% to 100%.
Take home message for trainees: Thymomas are malignant neoplasms. Stage and margin status are the most important prognostic parameters.
 adults with an incidence of 1-5 / million population / year. Thymomas often behave indolently, but are considered to be malignant neoplasms given their potential for aggressive local growth, recurrence, metastasis and potentially even might lead to the death of the patient. It is important to distinguish thymoma from thymic carcinoma, which has an overall worse outcome. Approximately 40% of patients with thymoma have a paraneoplastic or autoimmune syndrome of which myasthenia gravis is the most common. Conversely, 15 to 21% of patients with myasthenia gravis have a thymoma. These syndromes can even be the initial presenting factor as in this case. The prognosis of thymomas is mainly affected by margin status and tumor stage (TNM stage); histologic subtype is also associated with prognosis.
adults with an incidence of 1-5 / million population / year. Thymomas often behave indolently, but are considered to be malignant neoplasms given their potential for aggressive local growth, recurrence, metastasis and potentially even might lead to the death of the patient. It is important to distinguish thymoma from thymic carcinoma, which has an overall worse outcome. Approximately 40% of patients with thymoma have a paraneoplastic or autoimmune syndrome of which myasthenia gravis is the most common. Conversely, 15 to 21% of patients with myasthenia gravis have a thymoma. These syndromes can even be the initial presenting factor as in this case. The prognosis of thymomas is mainly affected by margin status and tumor stage (TNM stage); histologic subtype is also associated with prognosis. Thymomas are histologically divided according to the WHO classification into five main subtypes (A, AB, B1, B2, B3) based on the cytologic features of the epithelial tumor cells and the relative proportion of tumor cells and immature T-lymphocytes (thymocytes). Type A thymomas are comprised of bland-appearing spindle cells with only very few or no background lymphocytes; type B thymomas are comprised of polygonal epithelial cells. The type B thymomas are further subdivided with decreasing numbers of background lymphocytes from B1 to B3. Type AB is a mixture of type A and type B1 or B2 thymomas.

In the current case, gross evaluation (Figure 2) reveals a fleshy, lobulated, tan-pink solid and focally cystic mass with adjacent thymus tissue. Low power microscopic evaluation (Figure 8) shows a dense lymphoid infiltrate with a fibrous capsule and hypocellular fibrous septa. Also in figure 8, an area of paler tissue (arrow), a so-called “medullary island”, is identified along with Hassall corpuscles (Figure 9 arrow). Figure 10 shows scattered epithelioid cells (arrows). These morphologic features are consistent with a WHO type B1 thymoma.
The differential diagnosis of type B1 thymoma includes true thymic hyperplasia, thymic follicular hyperplasia, type B2 thymoma, T-lymphoblastic lymphoma (T-LBL), and thymic carcinoma. While true thymic hyperplasia is characterized by benign thymic parenchyma comprised of medulla and cortex, lacking the fibrous septa and fibrous capsule that are seen in thymomas, the weight of the thymic gland is above the weight that is normal for age. Follicular thymic hyperplasia is characterized by benign thymic gland that is expanded by lymphoid follicles with germinal centers. The differentiation of type B1 from B2 thymoma has historically been somewhat difficult as reflected by the only substantial reproducibility amongst thoracic pathologists. The 2015 WHO classification sought to address
 this issue by taking histologic criteria for each thymoma subtype and placing them into categories: “Obligatory/indispensable” or “optional.” The obligatory criteria for type B1 thymomas include abundance of immature T-cells, presence of medullary islands, and absence of epithelial cell clusters (these clusters are present in type B2). Optional criteria include presence of Hassall corpuscles or perivascular spaces. In T-LBL, blasts efface the normal thymic architecture without interspersed neoplastic epithelial cells. A keratin stain will be negative or highlights remaining thymic gland. In contrast to thymomas, thymic carcinomas have a distorted architecture and lack the typical lobulation of thymomas. In addition, they usually show at least focal desmoplasia and often have more pronounced cytologic atypia. While squamous cell carcinomas are the most common subtypes of thymic carcinomas, adenocarcinoma, sarcomatoid carcinomas, mucoepidermoid carcinomas, NUT carcinomas, and lymphoepithelioma-like carcinomas amongst others can also occur.
this issue by taking histologic criteria for each thymoma subtype and placing them into categories: “Obligatory/indispensable” or “optional.” The obligatory criteria for type B1 thymomas include abundance of immature T-cells, presence of medullary islands, and absence of epithelial cell clusters (these clusters are present in type B2). Optional criteria include presence of Hassall corpuscles or perivascular spaces. In T-LBL, blasts efface the normal thymic architecture without interspersed neoplastic epithelial cells. A keratin stain will be negative or highlights remaining thymic gland. In contrast to thymomas, thymic carcinomas have a distorted architecture and lack the typical lobulation of thymomas. In addition, they usually show at least focal desmoplasia and often have more pronounced cytologic atypia. While squamous cell carcinomas are the most common subtypes of thymic carcinomas, adenocarcinoma, sarcomatoid carcinomas, mucoepidermoid carcinomas, NUT carcinomas, and lymphoepithelioma-like carcinomas amongst others can also occur. Patients with type B1 thymoma have a favorable prognosis with 90% of tumors being completely resectable. Reported 10- and 20-year overall survival rates range from 85% to 100%.

Take home message for trainees: Thymomas are malignant neoplasms. Stage and margin status are the most important prognostic parameters.
References
den Bakker MA, Roden AC, Marx A, et al. Histologic classification of thymoma: a practical guide for routine cases. J Thorac Oncol 2014;9:S125-S130.
Marx A, Chan JKC, Coindre JM, et al. The 2015 WHO classification of tumors of the thymus: continuity and changes. J Thorac Oncol 2015;10(10):1383-95.
Roden AC, Yi ES, Jenkins SM, et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol 2015;10:691-700.
Roden AC, Yi ES, Jenkins SM, et al. Reproducibility of 3 histologic classifications and 3 staging systems for thymic epithelial neoplasms and its effect on prognosis. Am J Surg Pathol 2015;39:427-41.
Travis WD, Brambilla E, Burke AP, et al, editors. WHO classification of tumours of the lung, pleura, thymus and heart. 4th edition. Lyon (France): World Health Organization; 2015.
Marx A, Chan JKC, Coindre JM, et al. The 2015 WHO classification of tumors of the thymus: continuity and changes. J Thorac Oncol 2015;10(10):1383-95.
Roden AC, Yi ES, Jenkins SM, et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol 2015;10:691-700.
Roden AC, Yi ES, Jenkins SM, et al. Reproducibility of 3 histologic classifications and 3 staging systems for thymic epithelial neoplasms and its effect on prognosis. Am J Surg Pathol 2015;39:427-41.
Travis WD, Brambilla E, Burke AP, et al, editors. WHO classification of tumours of the lung, pleura, thymus and heart. 4th edition. Lyon (France): World Health Organization; 2015.
Contributors
Jordan Eldersveld, DO
Surgical Pathology Fellow
Department of Laboratory Medicine and Pathology
Mayo Clinic, Rochester, MN, USA
Anja C. Roden, MD
Associate Professor
Department of Laboratory Medicine and Pathology
Mayo Clinic, Rochester, MN USA
Surgical Pathology Fellow
Department of Laboratory Medicine and Pathology
Mayo Clinic, Rochester, MN, USA
Anja C. Roden, MD
Associate Professor
Department of Laboratory Medicine and Pathology
Mayo Clinic, Rochester, MN USA