Click here to see all images
April, 2020
Case of the Month
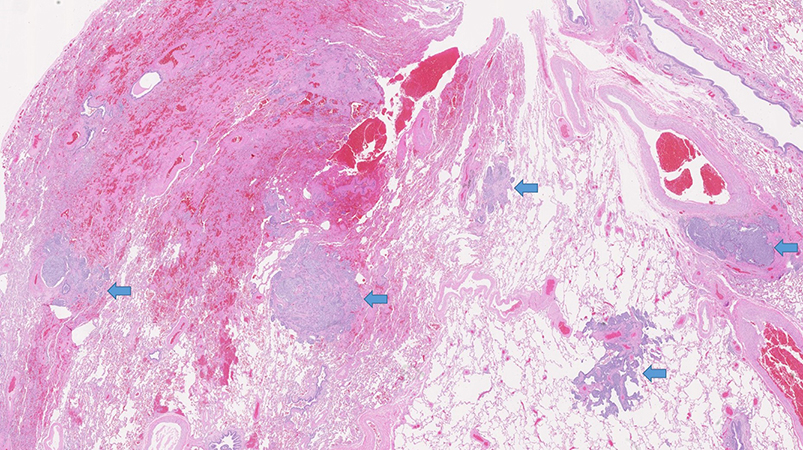
Clinical History: A 66-year-old woman with a history of resected pleomorphic sarcoma of the back status-post irradiation underwent surveillance imaging and was noted to have pulmonary nodules that were increasing in size. Chest CT showed numerous nodules in the left lower lobe (largest = 6 mm) and several smaller nodules in other lobes worrisome for metastatic disease. However, the nodules were not PET-avid. She underwent wedge resection followed by completion lobectomy (Figures 1-5). She is a non-smoker.
Quiz:
Q1. Which of the following is the best diagnosis based on these images?
- Neuroendocrine proliferations
- Metastatic pleomorphic sarcoma
- Melanoma
- Sarcomatoid carcinoma
Q2. The most likely immunohistochemical profile of these nodules is:
- Cytokeratins (-), TTF-1 (-), synaptophysin (-)
- Cytokeratins (-),TTF-1 (-), S-100 (+), MART-1 (+)
- Cytokeratins (+),TTF-1 (+), synaptophysin (+)
- Cytokeratins (+/-), TTF-1 (+/-), synaptophysin (+/-)
Q3. According to the current WHO classification, which of the following features best differentiate between typical carcinoid tumor and atypical carcinoid tumor?
- Mitotic rate and necrosis
- Ki-67 and p53
- Pleomorphism and lymph node metastasis
- Lymphatic invasion and spread through air spaces
Answers to Quiz
Q2. C
Q3. A
Diagnosis
Discussion
The spectrum of neuroendocrine proliferation in the lung ranges from neuroendocrine hyperplasia (clusters restricted to basement membrane), carcinoid tumorlets (< 5 mm) and carcinoid tumor (≥ 5 mm), mostly restricted to airways. The presence of isolated tumorlets is considered an incidental finding. However, multiple carcinoid tumorlets with diffuse neuroendocrine hyperplasia are seen most commonly in chronic lung disease, such as chronic bronchitis or emphysema as a reactive process, which may not lead to carcinoid tumor. Occasionally, in specimens containing a resected carcinoid tumor, neuroendocrine hyperplasia and tumorlets are seen in the background lung. Rarely, the neuroendocrine proliferation may cause small airway obstruction and peribronchiolar fibrosis leading to airflow limitation, progressing to respiratory failure. This syndrome of diffuse idiopathic neuroendocrine cell hyperplasia (DIPNECH) is considered preneoplastic due to progression to carcinoid tumors. The differential diagnosis includes minute meningothelial-like nodules, which are primarily differentiated from carcinoid tumorlets based on their perivenular location, presence of whorls and intranuclear inclusions, lack of fibrosis and absence of keratin/neuroendocrine markers. The other differential diagnosis is metastases as in this patient due to the presence of multiple lesions on imaging. This may lead to potential diagnostic pitfalls during intra-operative consultation. The presence of air trapping on expiratory CT-imaging along with multiple nodules would help the diagnosis in favor of DIPNECH. But up to 50% of patients with DIPNECH are asymptomatic for a long time. Sometimes, the interval between the onset of clinical symptoms and diagnosis may be more than a decade, which makes the diagnosis extremely challenging. Our patient has not developed any respiratory symptoms yet but remains on close clinical monitoring.
Take home message for trainees: Multiple pulmonary neuroendocrine proliferations may mimic metastatic lesions.
References
Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med 2010;134:1628-38.
Travis WD, et al. WHO classification of tumours of the lung, pleura, thymus and heart. WHO classification of tumours, 4th edition, volume 7, 2015.
Contributor
Assistant Professor of Pathology
Pulmonary Pathologist
Director, Immunohistochemistry
Department of Pathology
Virginia Commonwealth University
Richmond, VA