Click here to see all images
August, 2020
Case of the Month
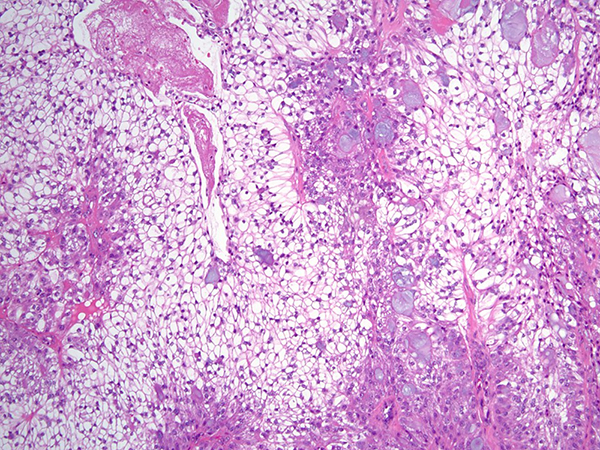
Clinical History: A 28-year-old man with progressively worsening dyspnea on exertion was noted on chest imaging to have a near-completely-obstructing endoluminal lesion in the distal trachea. He is a never smoker and endorsed no significant past medical history. Bronchoscopy confirmed the presence of a lobulated, partially-obstructing endotracheal lesion (Figure 1). A rigid bronchoscope was used to core out the lesion and electrocautery was applied to the base for tissue ablation and hemostasis. Histologic sections (Figures 2-5; H&E) demonstrated a circumscribed epithelial neoplasm composed of expansile nests of clear cells with only mild cytologic atypia. The clear cells were admixed peripherally with slightly larger-appearing cells characterized by eosinophilic cytoplasm and occasional small central nucleoli. Scattered throughout the tumor were collections of mucin-containing cells with occasional small cysts. A somewhat arborizing vascular network with some hyaline-appearing fibrosis was also present. Mitoses were not apparent and neither keratinization nor necrosis were identified. The over lying tracheal epithelium demonstrated early evidence of squamous metaplasia, but no overt cytologic atypia. Immunohistochemical stains (Figure 6) demonstrated the lesional cells to be diffusely CK7 positive with focal collections of cells demonstrating p40 and CK5/6 positivity. The lesional cells were TTF-1 negative.
Quiz:
Q1. Which of the following is true of this tumor?
- This tumor is more frequently seen in patients who endorse a history of tobacco smoking.
- About 50% of these tumors occur in individuals younger than 30 years of age.
- This tumor is more frequently seen in women.
- More often than not, this tumor carries a prognosis similar to other non-small cell lung cancers.
Q2. Which of the following ancillary tests would be most useful in confirming the diagnosis?
- Fluorescence in situ hybridization using probes targeted to the EWSR1 locus.
- Fluorescence in situ hybridization using probes targeted to the ALK locus.
- Fluorescence in situ hybridization using probes targeted to the MAML2 locus.
- EGFR mutational analysis by next generation sequencing.
Q3. The differential diagnosis for this case would reasonably include which of the following?
- Adenosquamous carcinoma
- Metastatic clear cell renal cell carcinoma
- Hyalinizing clear cell carcinoma
- PEComa
- All of the above
Answers to Quiz
Q2. C
Q3. E
Diagnosis
Discussion
The vast majority of recently published cases of PMEC harbor a recurrent t (11; 19) (q21; p13) translocation, resulting in a CRTC1-MAML2 fusion gene. In this case, fluorescence in-situ hybridization using probes to detect rearrangements of the MAML2 gene region was performed and reported as positive for rearrangement. Such rearrangements have been identified in both low and high-grade PMEC; though, their role as a prognostic indicator is not entirely clear. Immunohistochemically, PMECs are pancytokeratin, CK7, CK5/6, and p63/p40 positive. PMECs are TTF-1 and Napsin A negative. The presence of mucus cells and the MAML2 rearrangement effectively exclude the possibility of metastatic renal cell carcinoma. Hyalinizing clear cell carcinoma (HCCC) is also characterized by the presence of mucus cells; however, both salivary gland and pulmonary HCCC have been demonstrated to harbor EWSR1 rearrangements, not MAML2 rearrangements. Perivascular epithelioid cell neoplasms (PEComas) also lack MAML2 rearrangement and are generally positive for melanoma markers, smooth muscle actin, and desmin.
For this case, a final diagnosis of mucoepidermoid carcinoma, favor low-grade was rendered. Most PMECs are low-grade, and with complete surgical resection have an excellent prognosis. High-grade PMEC portends a prognosis similar to other non-small lung cancers. This patient subsequently underwent tracheal resection with lymph node dissection that revealed no residual disease or evidence of metastasis.
References
Salem A, Bell D, Sepesi B, et al. Clinicopathologic and genetic features of primary bronchopulmonary mucoepidermoid carcinoma: the MD Anderson Cancer Center experience and comprehensive review of the literature. Virchows Arch 2017;470:619-26.
Travis WD et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th Ed.
Contributor
Staff Pathologist
Department of Pathology
Cleveland Clinic
Cleveland, OH