Click here to see all images
July, 2020
Case of the Month
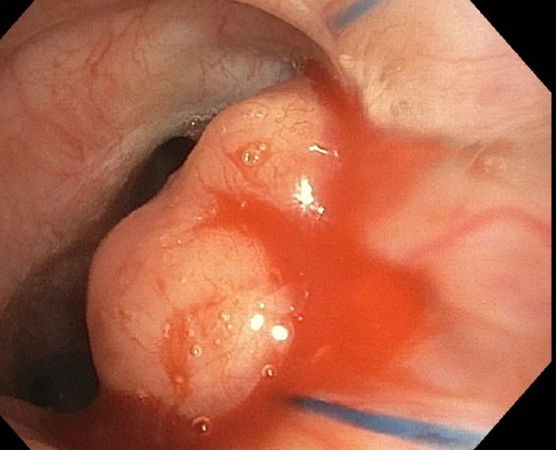
Clinical History: A 70-year-old woman status post bilateral lung transplant for interstitial lung disease was noted to have a 2.2-cm soft tissue nodular density at the medial margin of the left lung anastomotic suture site on chest computed tomography scan at six months. She was a former smoker. At bronchoscopy, the left lung anastomosis site showed an endobronchial nodule (Figure 1), which was biopsied (Figures 2-6; Figure 4 = iron stain; Figure 5 = von Kossa; Figure 6 = PAS). AFB stain and culture, and fungal cultures were negative, along with negative EBV and CMV by quantitative PCR and negative cryptococcal antigen serum test. Cultures of bronchoalveolar lavage fluid yielded “few Escherichia coli organisms”.
Quiz:
Q1. True or False? von Hansemann histiocytes with Michaelis-Gutmann bodies are pathognomonic of malakoplakia.
- True
- False
Q2. Conditions associated with malakoplakia include (select all that apply)
- Acquired immunodeficiency syndrome
- Organ transplantation
- Cytotoxic chemotherapy
- Sarcoidosis
Q3. Michaelis-Gutmann bodies are predominantly composed of inorganic components such as calcium, phosphorus and iron
- True
- False
Q4. The infectious agent usually detected in malakoplakia in thoracic transplant recipients is
- Escherichia coli
- Rhodococcus equi
- Stenotrophomonas maltophilia
- Pneumocystis jiirovecii
Answers to Quiz
Q2. A, B, C, D
Q3. B
Q4. A
Diagnosis
Discussion
The condition was first described by Michaelis and Gutmann in 1902, although first reported in 1901 by Professor von Hansemann. The urinary tract is the most commonly involved site, but there are increasing reports of malakoplakia occurring in a wide variety of other organs. An abnormal or altered immune response has been implicated in the pathogenesis of malakoplakia and is known to be associated with underlying conditions with immunosuppression, chronic prolonged illness, with cytotoxic chemotherapy or steroid use. Malakoplakia has been reported in solid organ transplant recipients, with renal transplant being the most common. In post-transplant settings, malakoplakia has been reported from early days of transplantation to up to fifteen years later. Recurrent infections and/or graft dysfunction are reported in up to 74% of renal transplant cases. Malakoplakia occurring post allogeneic hematopoietic stem cell transplant has also been reported.
Malakoplakia is associated with bacterial, mycobacterial and fungal pathogens, but Rhodococcus equi is the one most commonly identified in pulmonary malakoplakia in the setting of acquired immunodeficiency disease syndrome. In the setting of thoracic transplant recipients, malakoplakia has been reported with Escherichia coli as the underlying infectious organism, although Rhodococcus equi has also been recently reported in an allograft post-lung transplant presenting as a lung mass. Michaelis-Gutmann bodies and von Hansemann histiocytes are pathognomonic of malakoplakia. Michaelis-Gutmann bodies are reported to be composed of organic (94.6%) and inorganic (5.4%) components, the inorganic components being calcium, phosphorus and iron with weight percentages of 2.6, 2.1 and 0.7%, respectively.
Although data concerning standard treatment regimens are limited, the use of antibiotics that have intracellular action such as quinolones, trimethoprim-sulphamethoxazole, gentamicin and choline agonists that increase cyclic guanosine monophosphate (cGMP) levels have been shown to resolve the disease in most situations with good long-term prognosis. However, some refractory cases need surgical resection followed by prolonged antibiotic therapy.
Take home message: The diagnosis of malakoplakia in lung transplant recipients is important to recognize, as it might be mistaken clinically for primary malignancy or metastatic disease.
References
Kwon KY, Colby TV. Rhodococcus equi pneumonia and pulmonary malakoplakia in acquired immunodeficiency syndrome. Pathologic features. Arch Pathol Lab Med 1994;118:744-8.
Lane RJ, Kradin R, Xia D, et al. Malakoplakia in thoracic transplant recipients. Transplant Proc 2019;51:871-4.
Yousef GM, Naghibi B, Hamodat MM. Malakoplakia outside the urinary tract. Arch Pathol Lab Med 2007;131:297-300.
Contributor
Surgical Pathology Fellow
Department of Pathology and Laboratory Medicine
The Hospital of the University of Pennsylvania
3400 Spruce St, Philadelphia, PA, 19104
Charuhas Deshpande MD
Clinical Associate Professor
Department of Pathology and Laboratory Medicine
University of Pennsylvania
Founders 6.039
3400 Spruce Street
Philadelphia PA 19104