Click here to see all images
May, 2020
Case of the Month
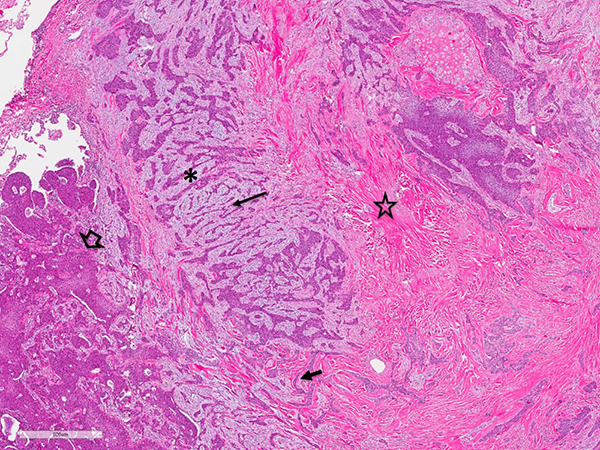
Clinical History: A 58-year-old male ex-smoker underwent bilateral lung transplantation for chronic severe COPD. He had an 80 pack-year history of smoking (quit in 2015). He also had a history of hypertension, severe osteoporosis with chronic vertebral compression fractures, and syndrome of inappropriate antidiuretic hormone (SIADH). He had previously undergone a chest CT, which showed a lobulated nodule with mild spiculation associated with - and obstructing - the right upper lobe apical subsegmental bronchus (Figure 1). Severe emphysema was also noted. The nodule had been present in follow up CT scans since it was first seen 4 years prior, and it had slowly increased in size to 1.3 cm. A PET/CT scan reported minimal increase of glucose uptake (SUV not available). Macroscopically, the right upper lobe nodule was homogeneous, tan-white, lobulated, and firm, and measured 1.3 x 1.2 x 1.0 cm. Figures include H&E (Figures 2-4), p40 and TTF-1 immunostains (Figure 5), and EWSR1 FISH using a break-apart probe (Figure 6).
Quiz:
Q1. One of the following statements is true for figure 6:
- This is an unsatisfactory FISH study.
- EWSR1 FISH shows a positive split signal, and helps to confirm the diagnosis.
- EWSR1 FISH is negative. FISH for MAML2 is required to confirm the diagnosis.
- This FISH study is inconclusive, and therefore does not help to make a diagnosis in this case.
Q2. Which of the following statements is correct?
- This tumor is most likely a metastasis from an abdominal organ.
- This type of pulmonary tumor is more frequently seen in the major salivary glands.
- Smoking is a risk factor for this tumor
- This tumor has indolent behavior with slow progression, but delayed local recurrence, and rarely late metastases, may occur.
Q3. Which of the following is the best diagnosis in this case?
- Metastatic renal cell carcinoma
- Squamous cell carcinoma
- Hyalinizing clear cell carcinoma
- Mucoepidermoid carcinoma
Answers to Quiz
Q2. D
Q3. C
Diagnosis
Discussion
Hyalinizing clear cell carcinoma (HCCC) is a rare low grade carcinoma of the head and neck, classically arising from the minor salivary glands. HCCC primary to the lung arising from the bronchial glands is extremely rare, as only 10 cases have been reported, five occurring in men and 5 in women. The patients’ ages ranged from 32 to 59 years old. Smoking history was provided for 7 patients, of whom 5 were smokers or former smokers. Pulmonary HCCC has similar morphologic features to its counterpart in the head and neck, and both also share the immunohistochemical and molecular profile. Head and neck HCCCs have an overall relatively good prognosis, with delayed local recurrences occurring in 10 to 20% of the cases. Rare cases with late distant metastases have been reported. All but one of the pulmonary cases did not have evidence of recurrence during follow-up periods that ranged from 10 to 181 months. In one case, the diagnosis of HCCC was made retrospectively on the resections of the recurrent lesions of a lung tumor that had been resected and diagnosed as squamous cell carcinoma 16 years prior.
Immunophenotypically, HCCCs show squamous differentiation, as they are consistently immunoreactive to cytokeratin 34βE12, p40, p63, and cytokeratin 5/6. The clear cells show PAS/PAS-D staining consistent with intracytoplasmic glycogen. HCCCs of the head and neck, and all reported pulmonary cases, show EWSR1-ATF1 rearrangements.
In view of the rarity of primary pulmonary HCCC and its histologic heterogeneity, this type of salivary gland tumor may not be easy to recognize. Differential diagnoses include squamous cell carcinoma with clear cell features, mucoepidermoid carcinoma with paucity of mucous cells, myoepithelial or epithelial-myoepithelial neoplasms, and clear cell carcinomas metastatic from extrathoracic sites, such as renal cell carcinoma. Differentiating HCCC from squamous cell carcinoma or mucoepidermoid carcinoma may be challenging, as they share squamoid morphology and squamous immunoprofile. Glands present in HCCC may mimic entrapped alveolar cells of a squamous cell carcinoma. Furthermore, mucous cells are not infrequently seen in HCCC. Therefore, recognition of the architecture and stromal characteristics of HCCCs is helpful to entertain this possibility in the differential diagnosis. The identification of the EWSR1 translocation with break-apart FISH, and absence of MAML2 rearrangements confirm the diagnosis of HCCC. Other differential diagnoses include myoepithelial and epithelial-myoepithelial tumors, which may be excluded with immunohistochemical stains, since HCCCs are negative for myoepithelial markers S-100, muscle-specific actin, smooth muscle actin, SOX 10 and calponin. Metastases from other sites should also be excluded using site-specific immunohistochemical stains. Last but not least, it is important to obtain a thorough clinical history to rule out metastatic HCCC from a previous head and neck primary.
Take home message: An unusual architectural pattern and prominent hyalinized fibrous stroma in a carcinoma with squamous differentiation are key features facilitating recognition of pulmonary hyalinizing clear cell carcinoma.
References
Jeffus SK, Gardner JM, Steliga MA, et al. Hyalinizing clear cell carcinoma of the lung: case report and review of the literature. Am J Clin Pathol 2017;148:73-80.
Takamatsu M, Sato Y, Muto M, et al. Hyalinizing clear cell carcinoma of the bronchial glands: presentation of three cases and pathological comparisons with salivary gland counterparts and bronchial mucoepidermoid carcinomas. Mod Pathol 2018;31:923-33.
Contributor
Assistant Professor
Pulmonary Pathologist
Department of Pathology
Cleveland Clinic, Cleveland OH