Click here to see all images
April, 2021
Case of the Month
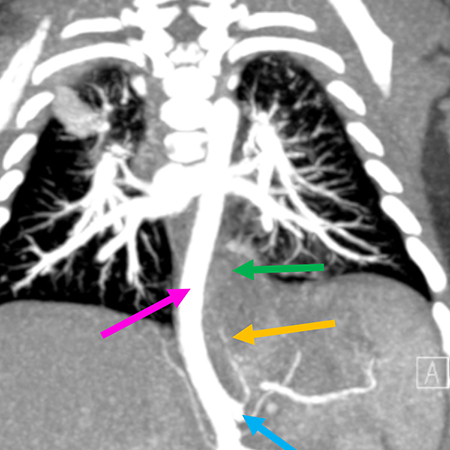
Clinical History: A 2-month-old male child was found to have a thoracic mass on prenatal ultrasound. Postnatal imaging was consistent with pulmonary sequestration. Computed tomography of the chest showed a 3.9-cm posterior mediastinal mass (Figures 1 and 2) extending into the infra-diaphragmatic region displacing the aorta without luminal compromise. Pulmonary sequestration was felt to be the most likely diagnosis, with retroperitoneal neuroblastoma as the less likely differential diagnostic consideration. The mass was surgically excised. H&E images are shown in Figures 3-6.
Quiz:
Q1. Which of the following germline mutations are seen in type 1 pleuropulmonary blastoma?
- STK11
- EGFR
- DICER1
- NCF-1
Q2. Which of the following leisons most commonly contains a type 2 congenital pulmonary airway malformation (CPAM)?
- Pulmonary sequestration
- Pulmonary hypoplasia
- Bronchial atresia
- Bronchopulmonary dysplasia
Q3. The presence of mucigenic epithelium in a congenital pulmonary airway malformation is strong evidence of which type of CPAM?
- Type 2
- Type 1
- Type 3
- Type 4
Answers to Quiz
Q2. A
Q3. B
Diagnosis
Discussion
Congenital pulmonary airway malformation (CPAM) is the most common type of congenital lung lesion, frequently diagnosed antenatally. Bronchopulmonary sequestration is the most common lesion in the differential diagnosis of CPAM, the former being defined by the absence of a connection to the tracheobronchial tree. In contrast to CPAM, which derives its blood supply from the pulmonary artery, the blood supply of sequestrations is derived from an anomalous systemic artery rather than the pulmonary circulation.
CPAM needs to be distinguished from pleuropulmonary blastoma and may rarely give rise to a mucinous adenocarcinoma (especially type 1 CPAM). The presence of non-neoplastic striated muscle fibers, described as “rhabdomyomatous dysplasia”, is extremely rarely seen in the lung. Associated cardiovascular and/or lung abnormalities have been described. Fraggetta reported striated muscle proliferation in type 2 CPAM and pulmonary sequestration. A rare case of pulmonary rhabdomyomatous dysplasia in a newborn with neurofibromatosis type 1 with no other pulmonary complications has also been described. The origin of striated muscle cells has been proposed as misplaced striated muscle from the pharynx, esophagus or diaphragm (i.e., a developmental error). Others favor the hypothesis of myoblastic differentiation/metaplastic transformation of primitive mesenchymal cells. In our case, neither lethal congenital malformations nor features of neurofibromatosis type 1 were present.
Take home message
Bronchopulmonary sequestrations are defined by systemic artery supply; they may harbor type 2 CPAM and skeletal muscle fibers.
References
Fraggetta F, Davenport M, Magro G, Cacciaguerra S, Nash R. Striated muscle cells in non-neoplastic lung tissue: a clinicopathologic study. Hum Pathol 2000;31:1477-81.
Hatanaka K, Yoshioka T, Tasaki T, Tanimoto A. Pulmonary rhabdomyomatous dysplasia of the newborn in neurofibromatosis type 1. Pathol Res Pract 2014;210:318-20.
Leblanc C, Baron M, Desselas E, et al. Congenital pulmonary airway malformations: state-of-the-art review for pediatrician's use. Eur J Pediatr 2017;176:1559-71.
Remberger K, Hübner G. Rhabdomyomatous dysplasia of the lung. Virchows Arch A Pathol Anat Histol 1974;363:363-9.
Contributors
Assistant Professor of Pathology
Pulmonary Pathologist
Director, Immunohistochemistry
Department of Pathology
Virginia Commonwealth University Health System
Richmond, VA, USA
Shaimaa A Fadl, MBChB
Assistant Clinical Professor
Cardiothoracic Imaging and Emergency Radiology
Department of Radiology
Virginia Commonwealth University Health System
Richmond, VA, USA