Click here to see all images
February, 2021
Case of the Month
Clinical History: A 60-year-old man with no significant past medical history, presented with several months of weight loss and fatigue. A chest CT revealed a 4 cm anterior mediastinal mass. The patient underwent resection of the mass. H&E images are shown in Figures 1-4. Results of immunohistochemical stains are in Figures 5 and 6.
Quiz:
Q1. Which statement is true regarding this tumor?
- The tumor maintains lobulated normal thymic architecture with internal fibrous septation
- Most patients with this tumor have a paraneoplastic syndrome
- Heavy smoking is a risk factor
- The mass is likely to contain foci of necrosis
Q2. The histopathologic features of this entity include all, except:
- Infiltrative sheets and islands of tumor cells
- Rare tumor cells may express neuroendocrine markers
- Numerous intermixed immature T-cells
- Broad zones of desmoplastic stroma
Q3. Most of these tumors express GLUT1
- True
- False
Answers to Quiz
Q1. D
Q2. C
Q3. A
Q2. C
Q3. A
Diagnosis
Thymic squamous cell carcinoma
Discussion
Thymic squamous cell carcinoma shows morphologic features similar to those of squamous cell carcinoma in other organs. Unlike thymoma, it often lacks a lobular architecture. Thymic squamous cell carcinoma accounts for the majority (~70%) of thymic epithelial malignant neoplasms and mostly occurs in the sixth decade. Males and females are almost equally affected. The etiology is unknown and there is no association with environmental factors, including cigarette smoking. About 1/3 of patients are asymptomatic at the time of presentation but others may have chest pain, cough and shortness of breath due to mediastinal compression. Paraneoplastic syndromes have been rarely reported. On imaging, TSCCs present as an anterior mediastinal mass and tend to have irregular borders with central areas of necrosis or cystic change. Tumors may invade the surrounding mediastinal structures, including the pericardium, lung, pleura, and major vessels. Regional lymph node and distant metastases are not uncommon.
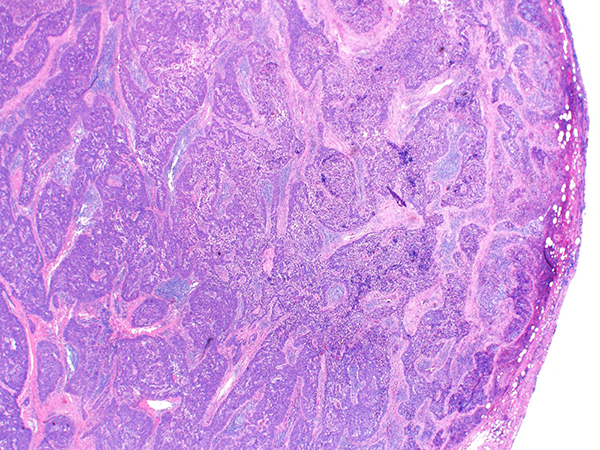
Grossly, the tumors range in size from 2-17 cm (mean 7 cm) and lack the encapsulation or internal fibrous septation seen in thymomas. Frequent foci of necrosis and hemorrhage may be seen. Histologically, the tumor is composed of sheets, nests, and cords of tumor cells with vesicular to hyperchromatic chromatin and distinct nucleoli (Figures 1-3) with associated chronic inflammation and desmoplastic stroma (Figure 4). Unlike thymoma, the intermixed lymphocytes are mature T cells and B cells. Terminal deoxynucleotidyl transferase (TdT)-positive immature T lymphocytes are absent (Figure 6). Tumor nests often have a smooth contour (Figure 2) but areas of infiltration with irregular borders are also found (Figure 4). By immunohistochemistry, thymic squamous cell carcinomas are immunoreactive for keratins, and most are positive for PAX-8 and p40 (Figure 5). Additional markers such as CD5, CD117 (Figure 5), GLUT1 and MUC1 are frequently expressed in thymic carcinomas, but are much less common in thymomas, and therefore may potentially be of value in the differential diagnosis of difficult cases. Focal expression of neuroendocrine markers is common in thymic carcinomas, but is rare in thymomas. The differential diagnosis also includes pulmonary squamous cell carcinoma, and therefore clinical and radiological correlation as well as CD5 and CD117 markers help in distinguishing thymic squamous cell carcinoma from pulmonary squamous cell carcinoma.
The 5-year survival in thymic squamous cell carcinoma is ~ 60%. The prognosis varies depending on completeness of resection, tumor size, lymph node status and overall stage.
Take home message: Thymic squamous cell carcinoma can be distinguished from type B3 thymoma by its infiltrative growth pattern, foci of necrosis, increased cytologic atypia, lack of TdT-positive lymphocytes and expression of CD5, CD117, GLUT1 and MUC1.
Grossly, the tumors range in size from 2-17 cm (mean 7 cm) and lack the encapsulation or internal fibrous septation seen in thymomas. Frequent foci of necrosis and hemorrhage may be seen. Histologically, the tumor is composed of sheets, nests, and cords of tumor cells with vesicular to hyperchromatic chromatin and distinct nucleoli (Figures 1-3) with associated chronic inflammation and desmoplastic stroma (Figure 4). Unlike thymoma, the intermixed lymphocytes are mature T cells and B cells. Terminal deoxynucleotidyl transferase (TdT)-positive immature T lymphocytes are absent (Figure 6). Tumor nests often have a smooth contour (Figure 2) but areas of infiltration with irregular borders are also found (Figure 4). By immunohistochemistry, thymic squamous cell carcinomas are immunoreactive for keratins, and most are positive for PAX-8 and p40 (Figure 5). Additional markers such as CD5, CD117 (Figure 5), GLUT1 and MUC1 are frequently expressed in thymic carcinomas, but are much less common in thymomas, and therefore may potentially be of value in the differential diagnosis of difficult cases. Focal expression of neuroendocrine markers is common in thymic carcinomas, but is rare in thymomas. The differential diagnosis also includes pulmonary squamous cell carcinoma, and therefore clinical and radiological correlation as well as CD5 and CD117 markers help in distinguishing thymic squamous cell carcinoma from pulmonary squamous cell carcinoma.
The 5-year survival in thymic squamous cell carcinoma is ~ 60%. The prognosis varies depending on completeness of resection, tumor size, lymph node status and overall stage.
Take home message: Thymic squamous cell carcinoma can be distinguished from type B3 thymoma by its infiltrative growth pattern, foci of necrosis, increased cytologic atypia, lack of TdT-positive lymphocytes and expression of CD5, CD117, GLUT1 and MUC1.
References
Travis WD, Brambilla E, Burke AP, et al, editors. WHO classification of tumours of the lung, pleura, thymus and heart. 4th edition. Lyon (France): World Health Organization; 2015.
Truong LD, Mody DR, Cagle PT, et al. Thymic carcinoma. A clinicopathologic study of 13 cases. Am J Surg Pathol 1990;14:151-66.
Zhao Y, Zhao H, Hu D, et al. Surgical treatment and prognosis of thymic squamous cell carcinoma: a retrospective analysis of 105 cases. Ann Thorac Surg 2013;96:1019-24.
Truong LD, Mody DR, Cagle PT, et al. Thymic carcinoma. A clinicopathologic study of 13 cases. Am J Surg Pathol 1990;14:151-66.
Zhao Y, Zhao H, Hu D, et al. Surgical treatment and prognosis of thymic squamous cell carcinoma: a retrospective analysis of 105 cases. Ann Thorac Surg 2013;96:1019-24.
Contributors
Mitra Mehrad, MD
Assistant Professor
Department of Pathology, Microbiology and Immunology
Vanderbilt University School of Medicine
Nashville, TN, USA
Assistant Professor
Department of Pathology, Microbiology and Immunology
Vanderbilt University School of Medicine
Nashville, TN, USA