Click here to see all images
February, 2022
Case of the Month
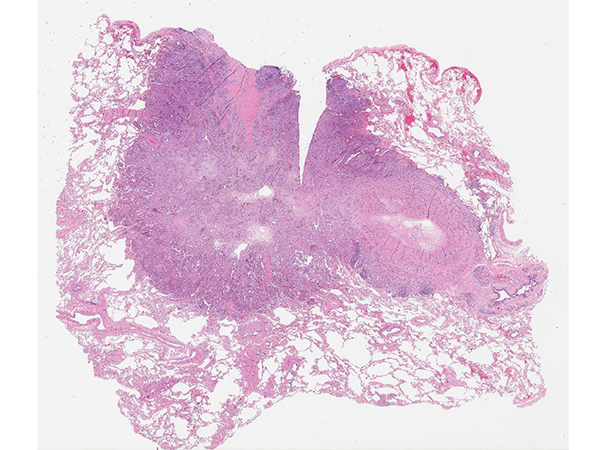
Clinical History: A 68-year-old man with 2 lung nodules underwent right middle lobectomy for a 2 cm nodule and a wedge resection of a 1 cm nodule in the right upper lobe. All hilar and mediastinal lymph nodes were negative. Photomicrographs from the right middle lobe nodule are shown in Figures 1 and 2. The right upper lobe nodule is shown in Figures 3 and 4.
Q1. How should this case be staged?
- pT4, since there are 2 nodules of adenocarcinoma in separate lobes on the same side
- pT3, since there are 2 nodules of adenocarcinoma in separate lobes on the same side
- pM1, since there are 2 nodules of adenocarcinoma in separate lobes on the same side
- Depends on the driver mutation status in the 2 tumors
Q2. If the driver mutation in each tumor is different, which of the following is the best interpretation?
- The 2 nodules are separate synchronous primaries
- This confirms that the correct stage is pT3
- This demonstrates clonal evolution in an intrapulmonary metastasis
- This confirms that the correct stage is pT4
Q3. If the driver mutation in both tumors is the same, what is the best interpretation?
- These 2 tumors cannot be separate synchronous primaries
- This proves that one tumor is a metastasis from the other
- This proves that the stage is pT4
- Depends on what the driver mutation is and its frequency; large-panel NGS can be helpful
Answers to Quiz
Q1. D
Q2. A
Q3. D
Q2. A
Q3. D
Diagnosis
Separate synchronous primaries of lung adenocarcinoma
Discussion
In this case, both lung adenocarcinomas were submitted for next-generation sequencing (NGS) for EGFR, KRAS, MET, BRAF, HER2, as well as testing for ALK (D5F3 immunohistochemistry), ROS1 (FISH) and RET (FISH). The adenocarcinoma in the right middle lobe was found to be wild-type (no driver mutation), while the adenocarcinoma in the right upper lobe had a KRAS driver mutation (KRAS c. 34G>T, p. Gly12Cys in exon 2). Based on this result, the 2 adenocarcinomas were diagnosed as separate synchronous primaries, and each was staged separately with its own synoptic template (pT1bN0 and pT1aN0).
There are 2 possibilities when you encounter 2 separate nodules of primary lung adenocarcinoma within the same lobe or in different lobes. One possibility is that one nodule is a metastasis from the other (i.e., the 2 nodules are “related”). Such cases are staged as intrapulmonary metastases using the pT3, pT4 or pM1 designation depending on the lobe in which the second nodule is located. However, the other possibility, which is common but often overlooked by trainees, is that both nodules could be separate synchronous primaries (“unrelated”). In such cases, the T3/T4/M1 designation is inappropriate. Traditionally, pathologists have attempted to differentiate between these 2 possibilities based on H&E morphology alone. However, in recent years, several investigators shown that molecular studies can help in the analysis (and staging) of such lesions.
A 2021 study by Bruehl et al. showed that in cases of 2 resected lung adenocarcinomas in which the H&E morphology of the 2 tumors was clearly different, this interpretation was supported by molecular analysis for driver mutations, which showed separate mutation status. However, in a subset of cases where the morphology of the 2 tumors was judged to be similar by H&E morphology, molecular analysis for driver mutations showed different driver mutation status, proving that the 2 lesions were unrelated, and showing that the H&E assessment of relatedness was inaccurate in this subset. It is clear from this and other studies that molecular analysis can supplement and improve the accuracy of traditional H&E morphology in this setting. Similar findings were reported recently by Pagan and colleagues (2020), and Ezer et al (2021).
In cases where 2 primary lung adenocarcinomas have different driver mutations (e.g., KRAS vs BRAF, KRAS vs. EGFR, or KRAS vs wild type), multiple studies have shown that these findings support the interpretation of separate synchronous primaries.
In cases where 2 primary lung adenocarcinomas have the same driver mutation or in cases where neither nodule has a driver mutation, interpretation is more complicated. In this setting, the interpretation can benefit greatly from large-panel NGS (if available), which allows analysis of numerous mutations and comparison of multiple mutations between the 2 tumors, rather than simply a single driver mutation. Such large-panel NGS testing, as recently demonstrated by Chang et al, can be helpful in resolving difficult cases.
The concept that the driver mutation in a given case of primary lung adenocarcinoma is retained in its metastasis is logical - in fact, the whole field of targeted therapy in metastatic lung adenocarcinoma is based on this premise. The most comprehensive study examining this issue was published in 2011 by Yatabe and colleagues. In this meticulous study, 50 lung adenocarcinomas with an EGFR mutation were divided into 3 parts, and each part was examined for EGFR mutation status. In each case, the EGFR mutation in each part from the same tumor was identical. Next, 5 EGFR-positive lung adenocarcinomas were divided into more than 100 pieces, each of which was tested for EGFR status. All pieces from the same tumor showed identical EGFR mutations. Finally, 77 pairs of EGFR-positive primary lung adenocarcinomas and their lymph node metastases were tested for EGFR. In all cases, the EGFR mutation was retained in the metastatic deposit.
Take home message for trainees: Do not assume that all resected multiple primary lung adenocarcinomas are T3, T4 or M1
There are 2 possibilities when you encounter 2 separate nodules of primary lung adenocarcinoma within the same lobe or in different lobes. One possibility is that one nodule is a metastasis from the other (i.e., the 2 nodules are “related”). Such cases are staged as intrapulmonary metastases using the pT3, pT4 or pM1 designation depending on the lobe in which the second nodule is located. However, the other possibility, which is common but often overlooked by trainees, is that both nodules could be separate synchronous primaries (“unrelated”). In such cases, the T3/T4/M1 designation is inappropriate. Traditionally, pathologists have attempted to differentiate between these 2 possibilities based on H&E morphology alone. However, in recent years, several investigators shown that molecular studies can help in the analysis (and staging) of such lesions.
A 2021 study by Bruehl et al. showed that in cases of 2 resected lung adenocarcinomas in which the H&E morphology of the 2 tumors was clearly different, this interpretation was supported by molecular analysis for driver mutations, which showed separate mutation status. However, in a subset of cases where the morphology of the 2 tumors was judged to be similar by H&E morphology, molecular analysis for driver mutations showed different driver mutation status, proving that the 2 lesions were unrelated, and showing that the H&E assessment of relatedness was inaccurate in this subset. It is clear from this and other studies that molecular analysis can supplement and improve the accuracy of traditional H&E morphology in this setting. Similar findings were reported recently by Pagan and colleagues (2020), and Ezer et al (2021).
In cases where 2 primary lung adenocarcinomas have different driver mutations (e.g., KRAS vs BRAF, KRAS vs. EGFR, or KRAS vs wild type), multiple studies have shown that these findings support the interpretation of separate synchronous primaries.
In cases where 2 primary lung adenocarcinomas have the same driver mutation or in cases where neither nodule has a driver mutation, interpretation is more complicated. In this setting, the interpretation can benefit greatly from large-panel NGS (if available), which allows analysis of numerous mutations and comparison of multiple mutations between the 2 tumors, rather than simply a single driver mutation. Such large-panel NGS testing, as recently demonstrated by Chang et al, can be helpful in resolving difficult cases.
The concept that the driver mutation in a given case of primary lung adenocarcinoma is retained in its metastasis is logical - in fact, the whole field of targeted therapy in metastatic lung adenocarcinoma is based on this premise. The most comprehensive study examining this issue was published in 2011 by Yatabe and colleagues. In this meticulous study, 50 lung adenocarcinomas with an EGFR mutation were divided into 3 parts, and each part was examined for EGFR mutation status. In each case, the EGFR mutation in each part from the same tumor was identical. Next, 5 EGFR-positive lung adenocarcinomas were divided into more than 100 pieces, each of which was tested for EGFR status. All pieces from the same tumor showed identical EGFR mutations. Finally, 77 pairs of EGFR-positive primary lung adenocarcinomas and their lymph node metastases were tested for EGFR. In all cases, the EGFR mutation was retained in the metastatic deposit.
Take home message for trainees: Do not assume that all resected multiple primary lung adenocarcinomas are T3, T4 or M1
References
Bruehl FK, Doxtader EE, Cheng YW, et al. Does histological assessment accurately distinguish separate primary lung adenocarcinomas from intrapulmonary metastases? A study of paired resected lung nodules and 32 patients using a routine next-generation sequencing panel for driver mutations. J Clin Pathol 2021; Online ahead of print. PMID 33649140
Chang JC, Alex D, Bott M, et al. Comprehensive next-generation sequencing unambiguously distinguishes separate primary lung carcinomas from intrapulmonary metastases: comparison with standard histopathologic approach. Clin Cancer Res 2019; 25:7113-25.
Ezer N, Wang H, Corredor AG, et al. Integrating NGS-derived mutational profiling in the diagnosis of multiple lung adenocarcinomas. Cancer Treat Res Commun 2021;29:100484.
Pagan CA, Shu CA, Crapanzano JP, et al. Correlation among morphology, next-generation sequencing/single gene analysis molecular testing, and clinical outcomes with eighth edition AJCC criteria. Am J Clin Pathol 2020;154:57-69.
Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol 2011;29:2972-7.
Chang JC, Alex D, Bott M, et al. Comprehensive next-generation sequencing unambiguously distinguishes separate primary lung carcinomas from intrapulmonary metastases: comparison with standard histopathologic approach. Clin Cancer Res 2019; 25:7113-25.
Ezer N, Wang H, Corredor AG, et al. Integrating NGS-derived mutational profiling in the diagnosis of multiple lung adenocarcinomas. Cancer Treat Res Commun 2021;29:100484.
Pagan CA, Shu CA, Crapanzano JP, et al. Correlation among morphology, next-generation sequencing/single gene analysis molecular testing, and clinical outcomes with eighth edition AJCC criteria. Am J Clin Pathol 2020;154:57-69.
Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J Clin Oncol 2011;29:2972-7.
Contributors
Sanjay Mukhopadhyay, MD
Director, Thoracic Pathology
Department of Pathology
Cleveland Clinic
Cleveland, OH, USA
Director, Thoracic Pathology
Department of Pathology
Cleveland Clinic
Cleveland, OH, USA