Click here to see all images
April, 2023
Case of the Month
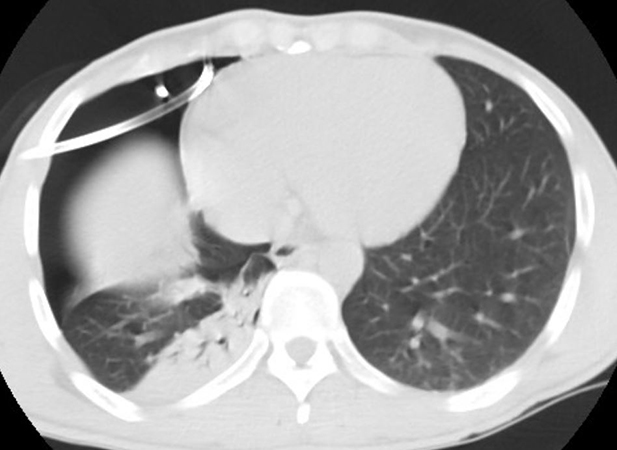
Clinical History: A 32-year-old man, non-smoker, was referred to our centre from an outside hospital for pleurodesis for unresolving spontaneous right pneumothorax despite chest tube placement. Preoperative CT scan confirmed the pneumothorax with proper chest tube placement, significant right lung atelectasis, and unremarkable left lung parenchyma (see Figure 1). Preoperative blood work showed a borderline eosinophilia (value of 0.5 x 10^9/L; upper limit of normal in our lab is 0.45). Intraoperatively the patient was found to have a right apical bullae, and underwent uncomplicated bullectomy and pleurodesis.
Specimens sent to pathology were a wedge resection of lung (7g, 4.5 cm in greatest diameter) and fragments of parietal pleura. A single subpleural bullae was noted grossly, without other significant abnormalities. Low power microscopic examination confirmed the subpleural bullae, on a backdrop of relatively normal appearing underlying parenchyma with the exception of rare interstitial non-necrotizing granulomas (Figure 2; star = bullae; circles= granulomas). At higher power, the wall of the bullae was characterized by a reactive pleuritis with mesothelial hyperplasia, histiocytes and eosinophils, with underlying fibroinflammatory remodelling, indicating some degree of chronicity (Figure 3). The granulomas consisted of a peripheral rim of epithelioid histiocytes and eosinophils, surrounding a structure consisting of a thin walled ovoid ring with a central body reminiscent of a multinucleated giant cell; these central structures were 70 to 80 µm in greatest dimension, had occasional spine-like projections (Figures 4 and 5). The structures had no significant refringence to polarized light and PAS-D positive (Figure 6).
Q1. All of the following are causes of eosinophilic pleuritis, except:
- Spontaneous pneumothorax
- Parasitic infection
- Talc pleurodesis
- Drug toxicity
Q2.The central structures within the granulomas are most consistent with:
- Aspirated plant material
- Schistosoma sp.
- Aspirated pill material
- Coccidioides sp.
Q3. Please select the best answer with regards to the central structures within the granulomas depicted in this case:
- They can be an incidental finding with no clinical symptoms
- They can be associated with pulmonary artery aneurysm
- They can be associated with underlying hepatic disease.
- All of the above
Answers to Quiz
Q2. B
Q3. D
Diagnosis
Discussion
While there is variability amongst species, the main histologic features of Schistosoma eggs are a relatively thin brown-greenish wall or shell surrounding multiple nuclei or miracidia. The subtyping of Schistosoma is often difficult on histologic preparations (as opposed to cytologic preparations), but is generally based on size of the eggs and location of the spine. The eggs elicit a eosinophilic granulomatous response. As the presence of schistosoma eggs in the lung are the result of hematogenous spread from the portal circulation, there is usually concomitant hepatic schistosomiasis, and pulmonary granulomas tend to be in a vascular distribution (although this was not easily appreciable in our case). It follows that vascular complications can occur as a complication of pulmonary involvement, including pulmonary artery aneurysm and pulmonary hypertension. In cases with heavy infection antischistosomal therapy can result in an acute eosinophilic Loeffler like syndrome from the massive release of parasitic antigens. As the parasite has the potential to seed to the spinal cord with severe neurologic complications, treatment is generally recommended.
The main entity in the differential diagnosis of Schistosoma is Paragonimus, as both have similar morphology and elicit the same type of eosinophilic granulomatous response. The eggs of Paragonimus are smaller (less than 100 µm in all cases), the wall/shell is thicker, lacks a well defined spine, and is generally birefringent. In some cases, particularly in the cases of smaller Schistosoma species (like S. japonicum) a definitive distinction on histology is not possible. Aspirated vegetable and/or pill material is generally refringent to polarized light and does not typically elicit an eosinophilic response. The cell wall of plants can resemble the wall of parasite eggs, however they do not contain the central nuclear structures nor the spine. The spherule of Coccidioides immitis can be similar in size to the eggs of Schistosoma (50-200 µm), but are morphologically round/spherical, lack a spine, and contain multiple mostly empty appearing endospores. The histologic reaction to C. immitis is most commonly a necrotizing granuloma.
Reactive eosinophilic pleuritis is a typical histologic manifestation of pneumothorax of any cause. Other more specific and rare causes include underlying eosinophilic pneumonia, drug toxicity (ex : methotrexate), and parasitic infection. Talc pleurodesis elicits an exuberant foreign body-type giant cell/granulomatous pleuritis that is not typically eosinophilic.
The clinical significance of Schistosoma in this particular case is difficult to determine. As there were no clinically or histologically evident vascular complications of infection, it could be that this is entirely an incidental finding. However, the lack of clinical symptoms does not negate the presence of viable organisms and the potential for more significant infection. The details on the place of birth and/or migration history of the patient were not available, however it can be assumed that they lived for some time in an endemic area. While there are two case reports of spontaneous pneumothorax in the setting of pulmonary schistosomiasis, a direct pathophysiologic link between the two has yet to be conclusively demonstrated. Following pleurodesis, this patient was lost to follow up at our center, but a referral for complete evaluation in microbiology was completed upon discharge.
This case underscores the necessity for close histologic examination of bullectomy specimens to determine an underlying etiology when possible, and for potentially significant incidental findings. In particular, reactive eosinophilic pleuritis is typical of spontaneous/idiopathic pneumothorax, eosinophilic granulomas are not, and their presence should prompt close examination of underlying cause with submission of additional tissue if need be.
Take home message for trainees: Think of schistosomiasis when you see foreign body like granulomas with eosinophils.
References
Feldman C, Kallenbach J, Sutej P, et al. Diffuse interstitial pulmonary fibrosis and spontaneous pneumothorax associated with Schistosoma haematobium infestation of the lungs. A case report. S Afr Med J. 69 (2); 1986 : 138-9.
Colley DG, Bustinduy AL, Secor WE, et al. Human schistosomiasis. The Lancet 383; 2014; 2253-2264.
Contributors
University of Montreal Health Center
Associate professor
University of Montreal Montreal
Quebec, Canada