Click here to see all images
January, 2023
Case of the Month
Clinical History: A 42-year-old woman presented with dyspnea with progressive deterioration since 9 months. She was afebrile but complained of a light dry cough without hemoptysis, a dry mouth without arthralgia and weight loss of 15kg. The exposure history was negative, and she was a non-smoker. At the time of diagnosis, she was taking the following medications: Fluoxetin 20 mg/d, Topiramate 25 mg 2cp/d, Perindopril/Indapamid 5/1.25 1x/d, Ibuprofen 400 3x/d Pantoprazol 20 mg/d Metformin 500 3x/d. Her previous medical history included chronic headaches due to idiopathic cranial hypertension and WHO stage II obesity (BMI 36.7 kg/m2). She also had a history of melanoma on the left arm, which had been completely resected without further treatment (pT1) two years ago. The CT scan showed multiple diffuse peribronchovascular and bilateral subpleural ground glass infiltrates predominantly affecting the upper lung fields, in a “crazy-paving” pattern. A bronchoalveolar lavage and a lung biopsy were performed. Representative images are shown (Figures 1 to 4).
Q1. What are the possible etiologies in the presence of such findings?
- Infectious
- Auto-immune
- Drug-induced
- Neoplastic
- All of the above
Q2. What type of special stains may help to refine the diagnosis?
- Periodic Acid Schiff
- May Grunwald-Giemsa
- Masson's trichrome stain
- Perls Prussian blue
Q3. What is the amorphous material composed of?
- Fibrin
- Necrosis
- Surfactant
- Amyloid
Q4. What is not a possible therapeutic strategy for such a condition?
- Rituximab
- Whole lung lavage
- Broad-spectrum antibiotics
- Wait and see
Answers to Quiz
Q2. A
Q3. C
Q4. C
Diagnosis
Discussion
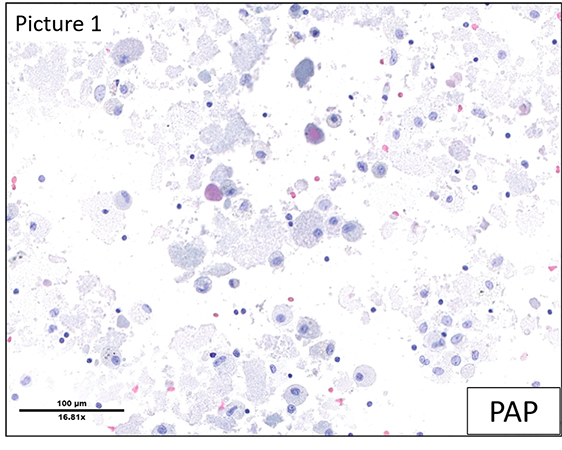
The H&E slide from the lung (Figure 4) shows the presence of finely granular proteinaceous material filling the alveoli (also PAS positive). There is no significant associated inflammation and there are no atypical cells.
The clinical differential diagnoses were hypersensitivity pneumonitis, eosinophilic pneumonitis, MALT lymphoma or Sjögren's syndrome, as well as drug-induced pathology or foci of alveolar hemorrhage. The histological differential diagnosis of an intra-alveolar exudate as seen in the present case comprises pneumocystis pneumonia, atypical mycobacterial pneumonia, pulmonary edema, and pulmonary alveolar proteinosis (PAP).
The morphological aspect and the PAS-positivity of amorphous material is characteristic of PAP. Based on the microscopic findings, an anti-GM-CSF (granulocyte-macrophage colony-stimulating factor) antibody test was performed, the positive result of which confirmed the autoimmune etiology of PAP in this case.
PAP is a very rare disease characterized by a malfunction of alveolar macrophages, causing intra-alveolar accumulation of surfactant proteins, impeding gas exchange. There are three broad categories of PAP: auto-immune (90% of cases), secondary (5-10%) and congenital (2%). Auto-immune PAP is defined by the presence of circulating anti-GM-CSF antibodies and is by far the most common cause. Secondary PAP is seen in patients with pre-existing conditions and precipitating factors such as hematological diseases including malignancies, inhalation of toxic substances or infections. Congenital PAP almost exclusively affects children and is caused by genetic disorders in surfactant production.
PAP is usually present in middle-aged adults (20-50 years old), particularly in smokers. The presentation is usually non-specific, with dyspnea or a minimally productive cough. Some cases may be asymptomatic.
A suggestive CT scan with typical crazy-paving pattern and a typical milky BAL with PAS-positive proteinaceous material are usually sufficient to make the diagnosis. Lung biopsy is sometimes required and shows alveoli filled with acellular eosinophilic deposits, with normal alveolar septa and no inflammatory infiltrate.
The clinical course of PAP is unpredictable and varies from spontaneous resolution to death due to respiratory failure or lung infection.
Treatment depends on the underlining cause. Whole lung BAL is performed to remove the alveolar material. Depending on the severity of the disease, alternative treatment options have been suggested, like GM-CSF injections and inhalations, plasmapheresis and Rituximab. Lung transplantation may be an option in few cases. Given that the efficacy of all these options is limited, a “wait and see” strategy may be considered, especially for mild or asymptomatic cases. Currently, the 5-year survival rate is 95%.
Take home message for trainees: Pulmonary alveolar proteinosis is a very rare diagnosis characterized by intra-alveolar, finely granular proteinaceous PAS- positive surfactant accumulation.
References
Patel SM, Sekiguchi H, Reynolds JP, Krowka MJ. Pulmonary alveolar proteinosis. Can Respir J 2012 Jul-Aug;19(4):243-5. doi: 10.1155/2012/841530. PMID: 22891182; PMCID: PMC3411387.
Borie R, Danel C, Debray MP, Taille C, Dombret MC, Aubier M, Epaud R, Crestani B. Pulmonary alveolar proteinosis. Eur Respir Rev 2011 Jun;20(120):98-107. doi: 10.1183/09059180.00001311. PMID: 21632797.
Ioachimescu OC, Kavuru MS. Pulmonary alveolar proteinosis. Chron Respir Dis 2006;3(3):149-59. doi: 10.1191/1479972306cd101rs. PMID: 16916009.
Contributors
Staff pathologist
Institute of Pathology
Lausanne University Hospital and University of Lausanne
Lausanne, Switzerland
Ekkehard Hewer, MD
Associate Professor
Institute of Pathology
Lausanne University Hospital and University of Lausanne
Lausanne, Switzerland
Sabina Berezowska, MD
Associate Professor Institute of Pathology
Lausanne University Hospital and University of Lausanne
Lausanne, Switzerland